In cereal grains and animal feed, Zearalenone (ZEN) is one of the worldwide most common mycotoxins and, consequently, humans and animals are at risk of being exposed to ZEN by consuming contaminated food products and feed. Due to the ethylenic double bond between C11 and C12 in the 14-membered macrocyclic lactone ring two stereoisomeric forms are generally possible, trans- and cis-ZEN. In different Fusarium species, only the trans-isomer could be isolated so far leading to the assumption that a highly isomer-specific pathway is involved in the fungal biosynthesis of ZEN. Toxicological studies revealed an elevated estrogenic activity of cis-ZEN and/or its reductive metabolites α/β-cis-Zearalenol compared to their respective trans-isomers.
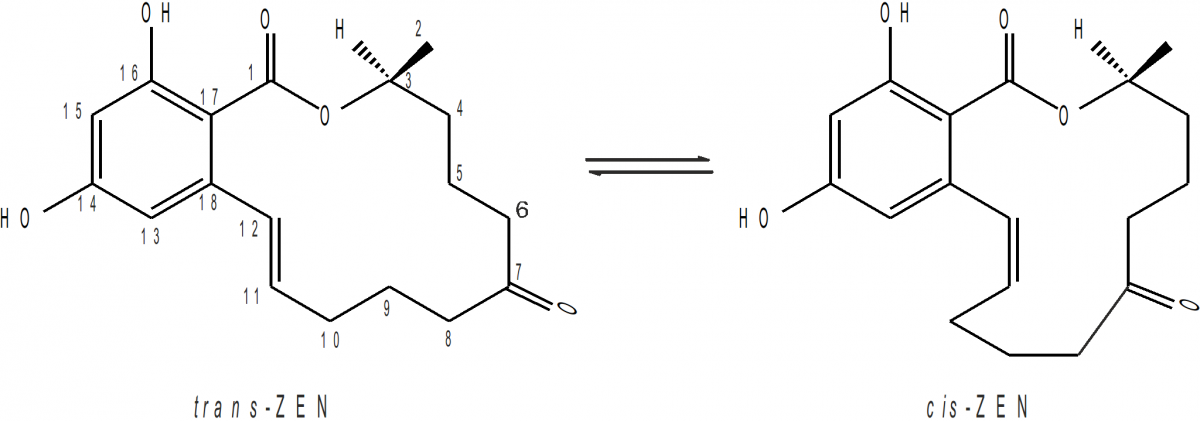 The non-steroidal mycotoxin Zearalenone (ZEN) is produced by a variety of Fusarium fungi. It was shown to be hyperestrogenic, hepatotoxic, haematotoxic, immunotoxic, genotoxic, teratogenic and carcinogenic. In cereal grains and animal feed, ZEN is one of the worldwide most common mycotoxins and, consequently, humans and animals are at risk of being exposed to ZEN by consuming contaminated food products and feed. Due to the ethylenic double bond between C11 and C12 in the 14-membered macrocyclic lactone ring two stereoisomeric forms are generally possible, trans- and cis-ZEN. In different Fusarium species, only the trans-isomer could be isolated so far leading to the assumption that a highly isomer-specific pathway is involved in the fungal biosynthesis of ZEN. Since the seventies, it is known that UV light irradiation as well as by sunlight yields a photochemical conversion of trans- to cis-ZEN. Reports on the occurrence of cis-ZEN as natural product may be due to exposure of the trans-isomer to light. Toxicological studies revealed an elevated estrogenic activity of cis-ZEN and/or its reductive metabolites α/β-cis-Zearalenol compared to their respective trans-isomers.
The non-steroidal mycotoxin Zearalenone (ZEN) is produced by a variety of Fusarium fungi. It was shown to be hyperestrogenic, hepatotoxic, haematotoxic, immunotoxic, genotoxic, teratogenic and carcinogenic. In cereal grains and animal feed, ZEN is one of the worldwide most common mycotoxins and, consequently, humans and animals are at risk of being exposed to ZEN by consuming contaminated food products and feed. Due to the ethylenic double bond between C11 and C12 in the 14-membered macrocyclic lactone ring two stereoisomeric forms are generally possible, trans- and cis-ZEN. In different Fusarium species, only the trans-isomer could be isolated so far leading to the assumption that a highly isomer-specific pathway is involved in the fungal biosynthesis of ZEN. Since the seventies, it is known that UV light irradiation as well as by sunlight yields a photochemical conversion of trans- to cis-ZEN. Reports on the occurrence of cis-ZEN as natural product may be due to exposure of the trans-isomer to light. Toxicological studies revealed an elevated estrogenic activity of cis-ZEN and/or its reductive metabolites α/β-cis-Zearalenol compared to their respective trans-isomers.
Quantum-chemical computations of electronic densities and pKa values
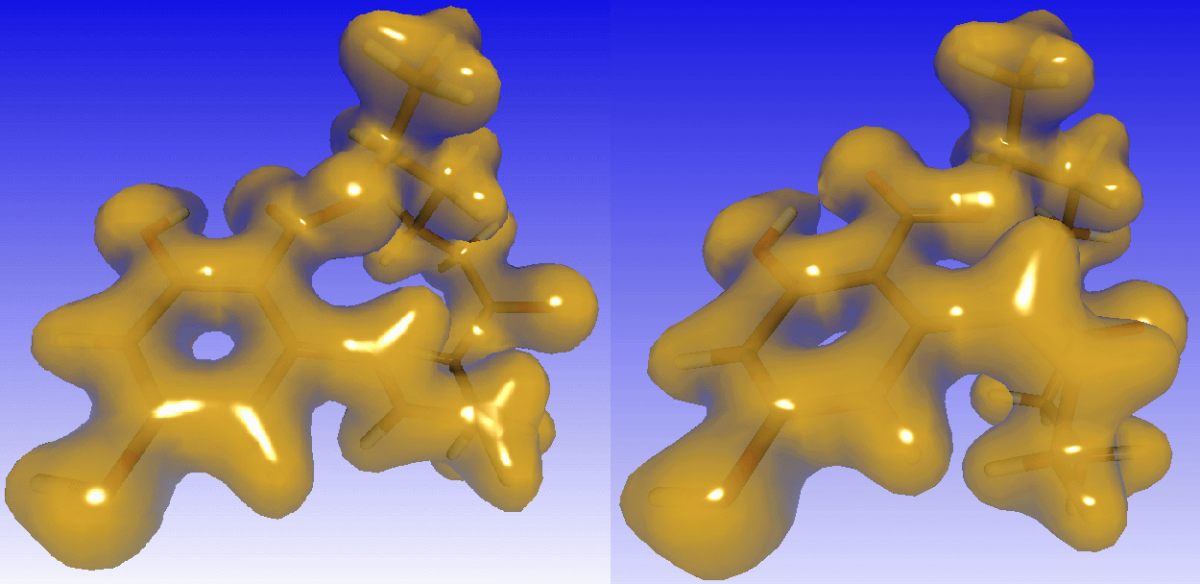 In contrast to cis-ZEN, trans-ZEN shows a clearer boundary between the electrons' locations of the C16-hydroxy hydrogen and the C1 carbonyl oxygen as depicted in the electron desities computed with Gaussian indicating keto-enol tautomerism and suggesting resonance-stabilization of both involved oxygens of cis-ZEN. This assumption was confirmed by the estimation of pKa values for both ZEN isomers as well as for two molecules obtained by transferring the C16 hydroxy hydrogens to the respective C1 carbonyl oxygen. With 7.69 and 7.67, the pKa difference between cis-ZEN and its modified form is indeed negligible, whereas the values computed for trans-ZEN and its tautomeric counterpart differ considerably with 7.43 and 7.18, respectively. Hence, the cis-isomer of ZEN has more equally likely tautomeric end states than trans-ZEN which prefers residing in one state only revealing a significantly larger entropy of cis-ZEN, possibly explaining its higher concentration at equilibrium.
In contrast to cis-ZEN, trans-ZEN shows a clearer boundary between the electrons' locations of the C16-hydroxy hydrogen and the C1 carbonyl oxygen as depicted in the electron desities computed with Gaussian indicating keto-enol tautomerism and suggesting resonance-stabilization of both involved oxygens of cis-ZEN. This assumption was confirmed by the estimation of pKa values for both ZEN isomers as well as for two molecules obtained by transferring the C16 hydroxy hydrogens to the respective C1 carbonyl oxygen. With 7.69 and 7.67, the pKa difference between cis-ZEN and its modified form is indeed negligible, whereas the values computed for trans-ZEN and its tautomeric counterpart differ considerably with 7.43 and 7.18, respectively. Hence, the cis-isomer of ZEN has more equally likely tautomeric end states than trans-ZEN which prefers residing in one state only revealing a significantly larger entropy of cis-ZEN, possibly explaining its higher concentration at equilibrium.
Steady state distribution of E-/Z-ZEN interconversion by classical force field simulations
ZEN isomers have been simulated with both the amber and the Merck molecular force field. In addition, their artificial derivatives with smaller lactone rings were investigated. These were built by successively omitting ring atoms together with their substitutes/hydrogens starting with C10 followed by C9, C8, …, and C5 according to the atom labeling shown above. Mean and minimal potential energies have been computed for all (2 x 7 = 14) molecules in order to see the enthalpic/sterical effect on the equilibrium distribution of trans- and cis-ZEN after interconversion. Both force fields demonstrate that for small cycloalkenes the cis-isomer is favored, i.e. the thermal equilibrium is supposed to be dominated by the cis-isomer which is somewhat intuitive.
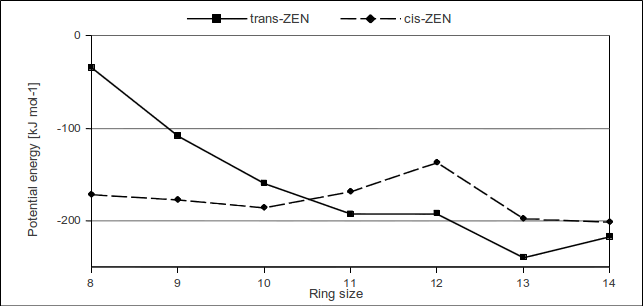
However, with increasing ring size, the trans-isomer becomes more favorable. According to these results which strongly correlate with respective mean potential energies, the trans-isomer is preferred by cyclic molecules with a ring size larger or equal to ten or eleven atoms depending on the utilized force field. As the ring grows further, its steric effect on the potential energy becomes more and more negligible, yielding smaller differences between the two isomers. On the basis of these enthalpic entities resulting from classical force field simulations, it is not possible to explain the substantially higher preference of cis-ZEN to the trans-isomer. This observation as well indicates the dominance of cis-ZEN due to entropic reasons.