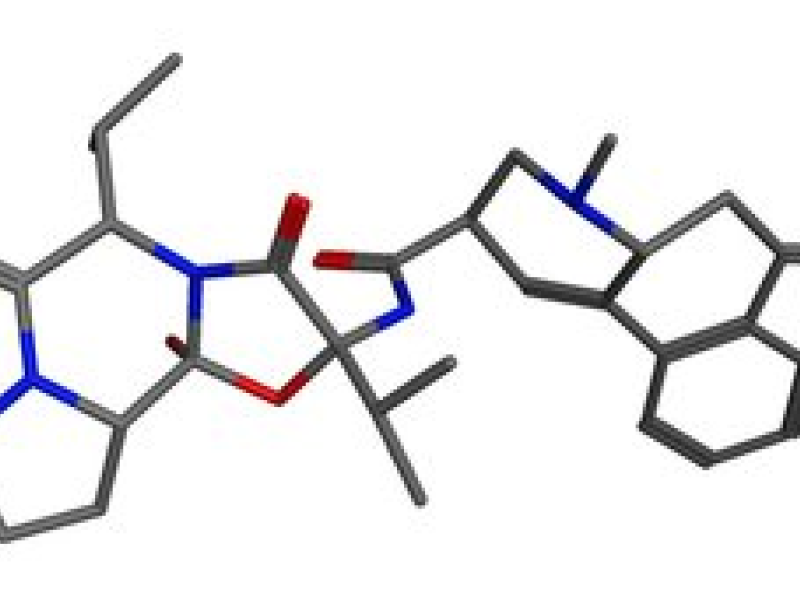
We investigated six chemical agents of the group of alkaloid ergotamines. All substances are derivatives of lysergic acid. This kind of alkaloid can be found in fungi like Claviceps purpurea. When consumed in larger doses this causes ergotism, inducing a type of gangrene especially in the extremities. All molecules can exist in three different configurations, as an R- or S-epimer or as a transition state. The latter is called an enol-intermediate.
The R-epimer of Ergocornin is depicted below:
The molecules have been optimized using the software-package zibgridfree. Thereafter each agent was immersed in a solution consisting of either water or acetonitrile or both. Furthermore the same line of action was done for the protonated molecules in order to account for different pH-values. The next step was to determine the interaction energy between the solvent and the optimized solute. In general the calculated inner energies cannot be correlated with the experimental findings, the only exception are the simulations of the alkaloids, which are dissolved in a mixture of water and acetonitril. However these correlations are not systematic. From out current point of view the pure treatment of energies is not suitable for a concise interpretation of the experimental data, particularly with regard to the very low energies of the transition states.
But in geometric terms, a classification of the R- and S-epimers is easily constructed. This concept can be applied to explain the preference of the S-epimers in the experiments. One can see that the R-epimers are much closer to a flat structure (i.e. the transition state) than the S-epimers. Therefore the R-epimers are more similar to the transition state and epimerize easier. Henceforth, the equilibrium is shifted towards the S-epimers. That is the two free energy minima are separated by a kinetic barrier to epimerization. The conformations of the R-epimers are lower in entropy, but stabilized by enthalpy, mostly through aromaticity. The S-epimers are higher in entropy and are trapped in a higher energetic state by an entropic bottleneck. The calculated dihedral angles clarify this connection.
Besides an energetic and a kinetic point of view, certain alkaloid ergotamines can undergo pi-stacking-interactions, for instance ergotamin and ergocristin. Only S-epimers can show aromatic-aromatic interactions but not the R-epimers. In the former state, the benzol ring is adjusted perpendicularly to the aromatic base frame. This means a further lowering in energy, especially in water. In the end, the S-epimer will be more stabilized with a smaller concentration of acetonitrile. Yet, another example for a steric argument.