Analgesics, e.g. morphine, are widely used for pain treatment. By binding to the body's own µ-opioid receptor, pain-relieving effects are reached. It is already known that analgesics have a higher effect in inflamed tissue than in healthy tissue, but there are still various side effects like nausea and addiction. The aim of this project is to analyze the interactions between morphine (drug) and the μ-opioid receptor (receptor), and find possible reasons for the different behavior in healthy and inflamed tissue. For instance, the differing pH-value, conformational changes of the receptor in the inflamed tissue or diverging transmembrane potentials could influence the stronger effect. By studying the μ-opioid receptor these conjectures have to be verified or falsified to hopefully, later on, develop analgesics that are acting in the inflamed tissue only. For this investigation, both molecular simulations and chemical experiments are used.
Molecular simulation
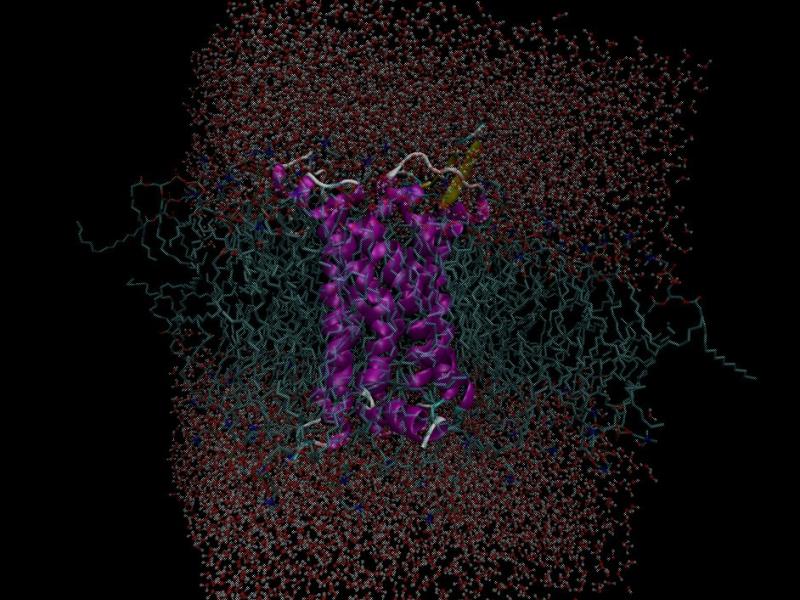
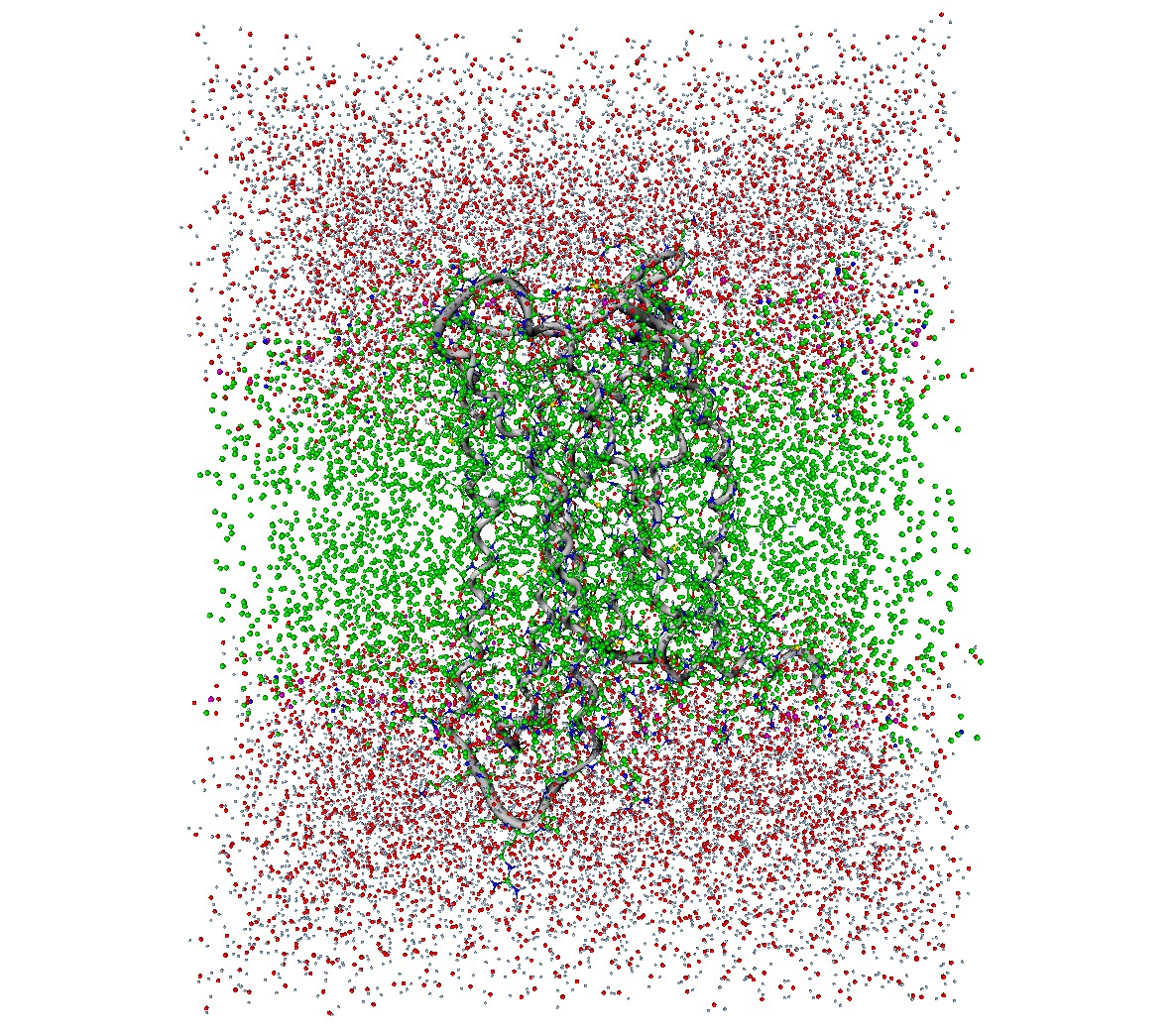
On the basis of the crystal structure of the μ-opioid receptor bound to a morphinian antagonist found in 2012 by Manglik et al. a molecular model of the receptor was created. In preparation for the simulation the molecule had to be parametrized and put into a system of a lipid bilayer and water. Since the normal force fields of Gromacs cannot handle both proteins and lipids, Berger et al. derived the Berger Lipids which are the most widely-used parameters for the lipid part of membrane simulations. As lipid bilayer the DPPC (Dipalmitoylphosphatidylcholine) belonging to the phospholipids was chosen and packed around the protein. The following solvation with water was a bit tricky since gaps in the lipid acyl chains might be filled with random water molecules. By increasing the van der Waals radius of carbon the addition of water within the lipids gets less likely, so that the few stray molecules can be deleted manually. After adding ions to neutralize the system, minimization of energy to exclude inappropriate geometry, and equilibration to stabilize the temperature and the pressure of the system, the simulation box consisting of 31722 atoms can be used for molecular dynamics with Gromacs.
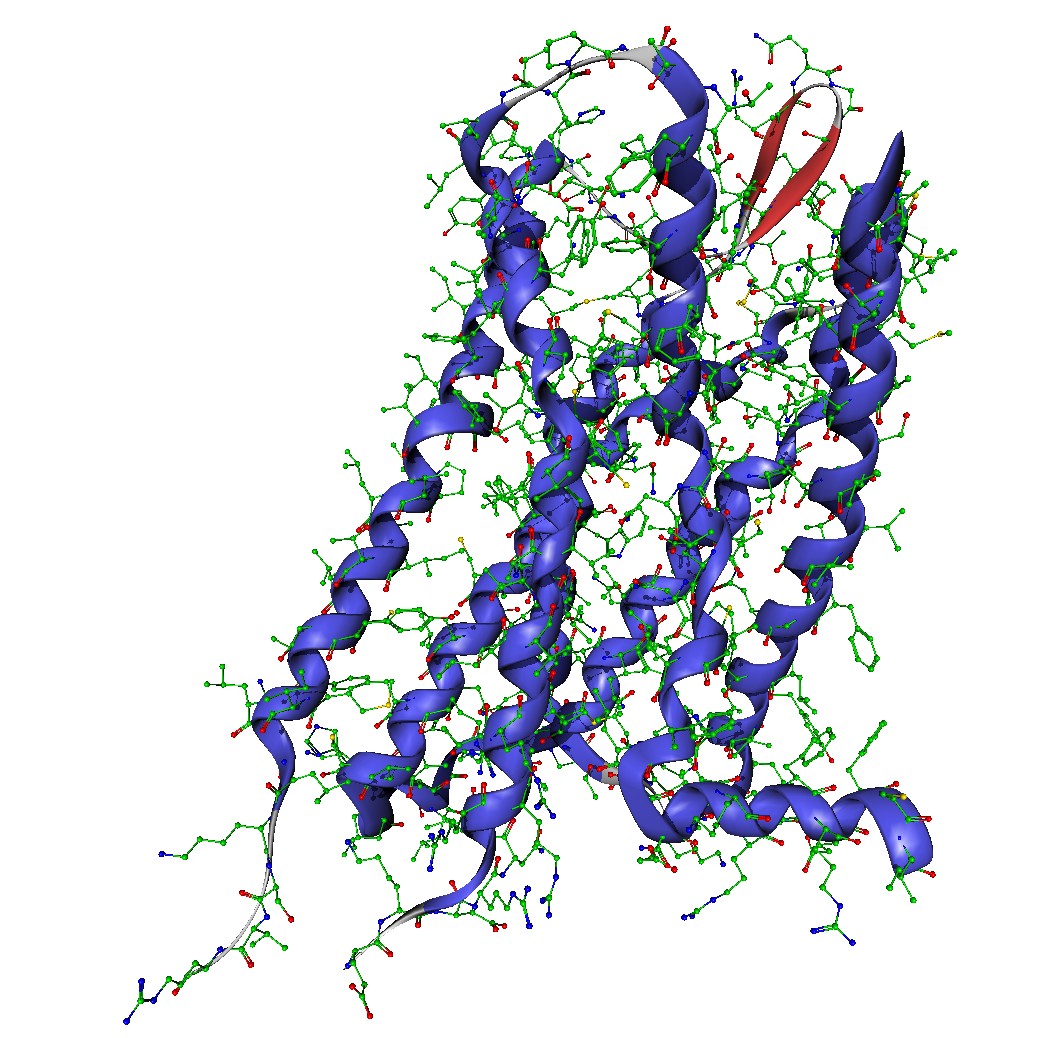
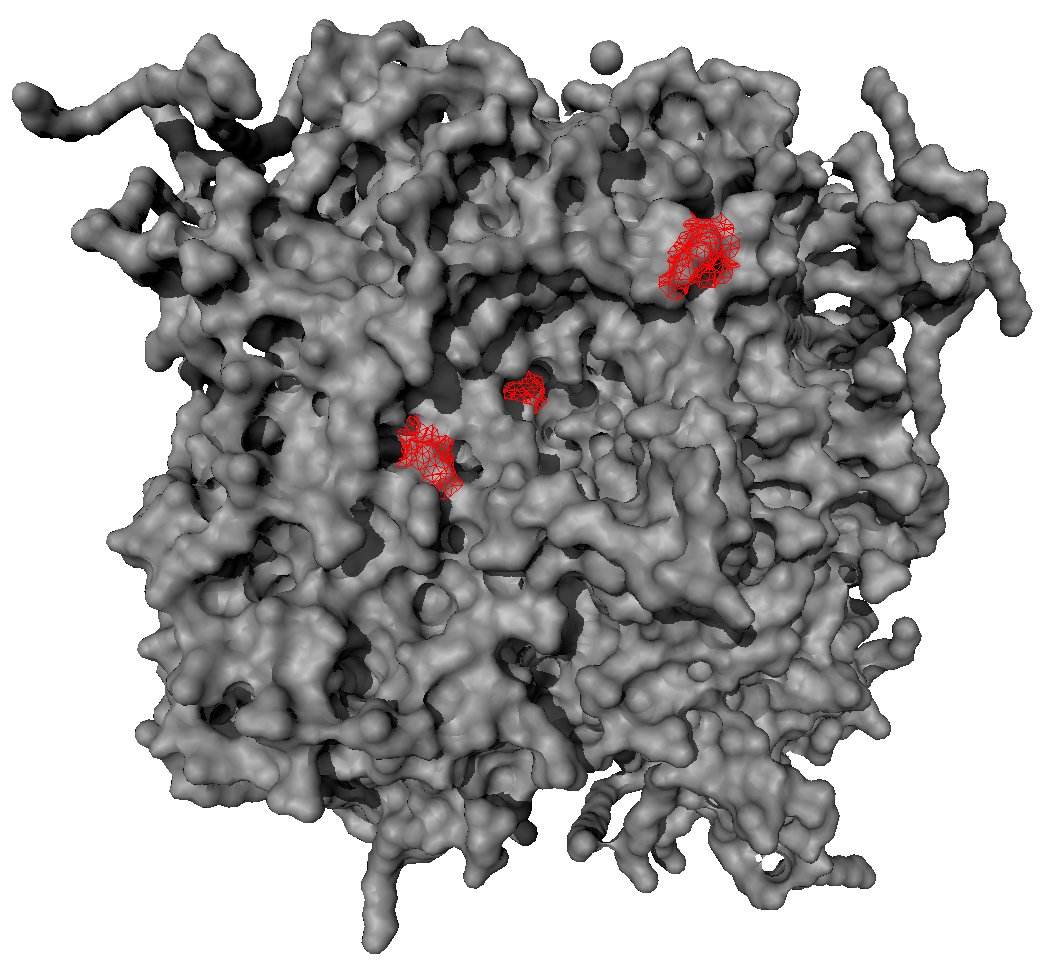
Since the task is to compare the different behavior of morphine in healthy and inflamed tissue, the receptor has to be modelled in inflamed tissue as well. Therefore the pH-value is important, which is about 7.4 in healthy tissue and approximately 5.5 in inflamed tissue. This means, that amino acids with a pKa-value between 5.5 and 7.4 have to be protonated, which is only the case for histidine. Considering the binding pocket, three histidines are lying in the center or the nearby area, which is a good indicator for the higher effect of morphine in inflamed tissue.
Since proteins are denaturing at high temperature so that the system drifts apart, a high-temperatured presampling to discover the conformation space of the μ-opioid receptor is not possible. By simulating at low temperature and starting in only one conformation the simulation will probably not overcome energy barriers to find other conformations, even not over a long time. Therefore, several starting points for separate samplings have to be generated. In the group of Frank Noé (Freie Universität), the conformation dynamics of rhodopsin were studied, and a huge amount of simulation data was gained. Like the μ-opioid receptor, rhodopsin belongs to the family of rhodopsin-like G-protein-coupled receptors and possesses seven transmembrane helices. There are different possibilities to use the approximately 8000 identified cluster centers of rhodopsin as starting structures for the μ-opioid receptor: on the one hand the homology modeling based on a sequence alignment, on the other hand using the hierarchical relevant descriptor detector, by restraining the most relevant descriptors and equilibrating this system at low temperature [2012: A Coarse Graining Method for the Dimension Reduction of the State Space of Biomolecules]. From these configurations many parallel running MD trajectories can be started to interconnect single snapshots by simulation, and to get from single states to processes. Finally using free energy calculations [2011: Direct Reweighting Strategies in Conformation Dynamics] the question about the different behavior of morphine in inflamed tissue will hopefully be solved.
Chemical experiments
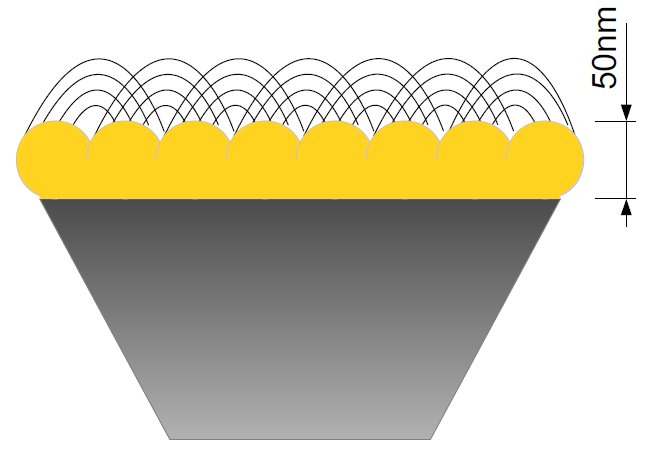
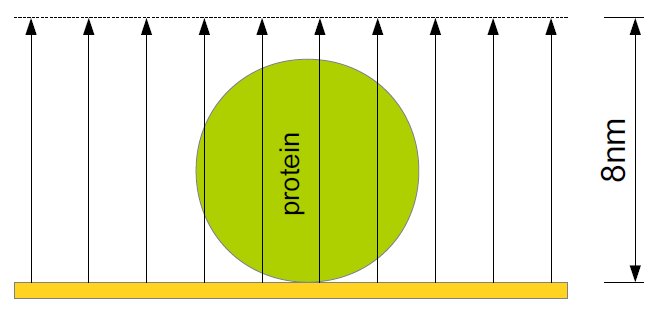
The chemical experiments in collaboration with the group of Prof Hildebrandt at Technische Universität Berlin are based on the ATR-IR method (attenuated total reflection infrared spectroscopy) which allows the examination of liquid samples. The IR radiation is projected on a prism of silicium which results in an evanescent wave. This wave typically goes 1μm deep into the sample. By coating the prism with a 50nm thick nanostructured gold film, a depth of only 8nm can be achieved. This surface-enhancement is called SEIRA (surface-enhanced infrared absorption spectroscopy) and allows the study of monolayers. The IR radiation generates an electrical field above the gold film that is nearly perpendicular within the first 8nm, which leads to an enhancement of intensity and a perpendicular polarization. The latter one means that vertical vibrations are measured with a higher intensity so that statements on the orientation can be made. Additionally the gold film is used for the binding of the protein within the membrane so that the protein is within a preferably native environment and bound to the surface. Thereby, the advantages comprise the demand of only small amounts of the protein and possible modifications at the same mono-film, like changing the pH-value or adding ligands.
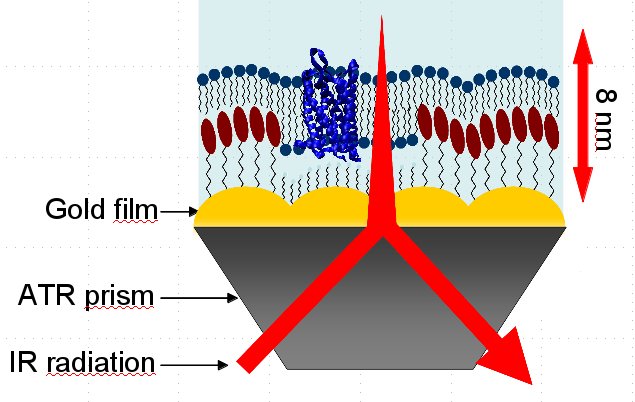
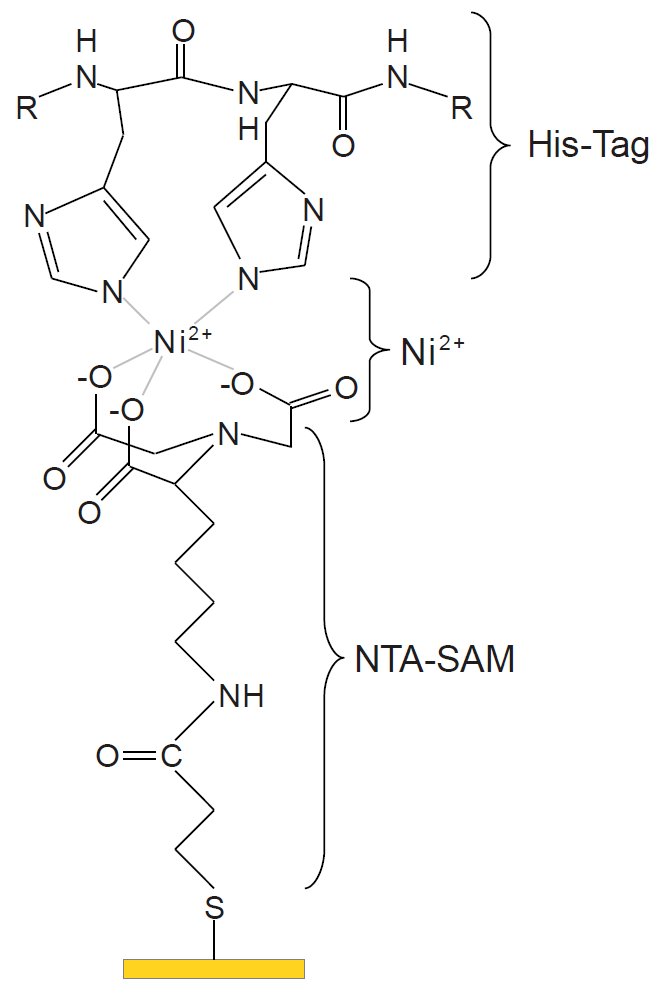
First the gold film is built. Via sulfur a self-assembled monolayer (SAM) of Nitrilotriacetic acid (NTA) is bound. Nickel ions are bound to the NTA molecules, where the His-Tag of the protein is bound to. In the first step, the protein is in a detergent. Here, the right buffer has to be chosen so that the pH-value is constant in a preferably large range and the system is stable. Adding vesicles a membrane is constituted. Thereby the critical micelle concentration (CMC) has to be regarded, since a too little concentration, e. g. because of additional buffer, is leading to a dissolving of the micelles and, thus, a damage of the protein.