The research project TransRisk as a sub-project of the joint project RiSKWa funded by the Federal Ministry of Education and Research deals with the characterization, communication and minimization of risk emerged from new pollutants in the water cycle. In particular, our working group will support the identification of harmful transformation products arising from degradation of anthropogenic substances.
Organisation of the project
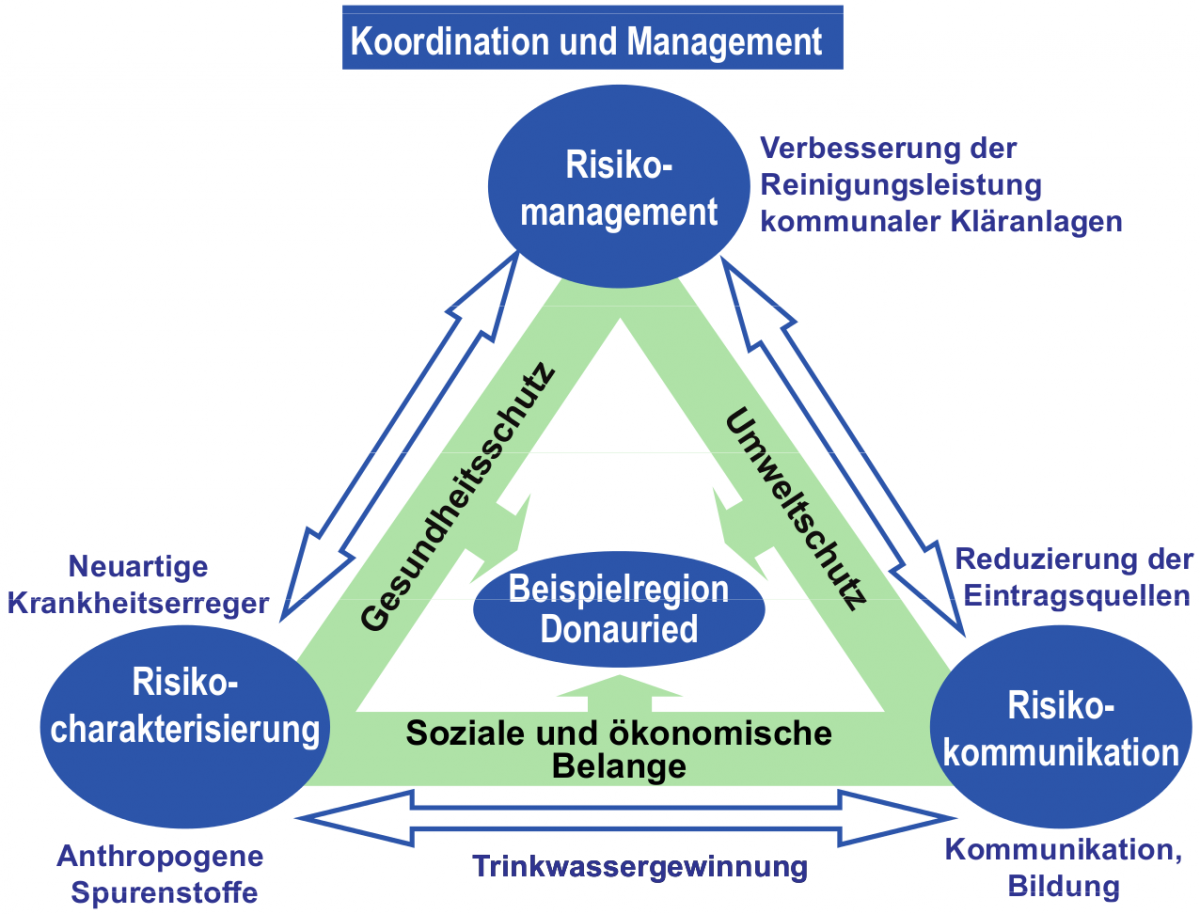
Usually, when developing new compounds, only the produced substance itself undergoes a risk assessment. Chemicals arising upon degradation or metabolization of the parent molecule, so-called transformation products (TP), are not considered, altough they might cause more harm than the parent compound itself. From a toxicologic point of view, there is clearly a need for studying the TP object space in order to reduce the risk of novel toxicologic effects. The project is divided into the sections risk characterization, risk management, and risk communication dealing with the identification of novel pollutants, the improvement of wastewater treatment, and the communication between participants as well as with media, respectively. The region Donauried including a purification plant in southern Germany was selected as a model for the technical application of new toxicologic insights.
In recent years, first and higher order (TPs) besides their parental compounds increasingly attracted the attention of human and ecotoxicologists. Due to an increasing number of novel TPs, a risk assessment and prioritization by means of in silico methods becomes more and more indispensable. The limited resources of time-consuming and expensive laboratory experiments including the development of a synthesis protocol as well as in vitro and in vivo tests for toxicity quantification can then be restricted on metabolites that are most likely to affect critical biological targets. Due to structural similarities between parent compounds and their low order degradation products, one is typically interested in the question whether TPs are able to bind to the same targets as known for the parent compound.
Risk characterization
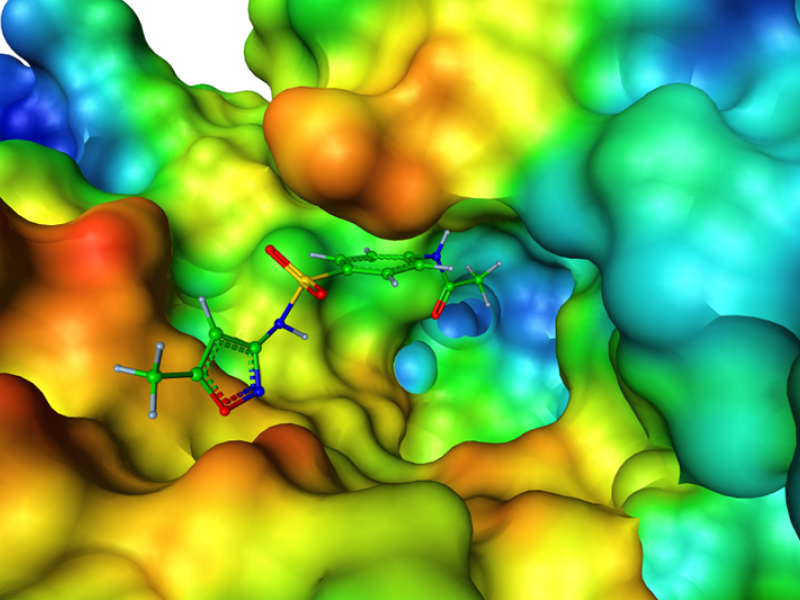
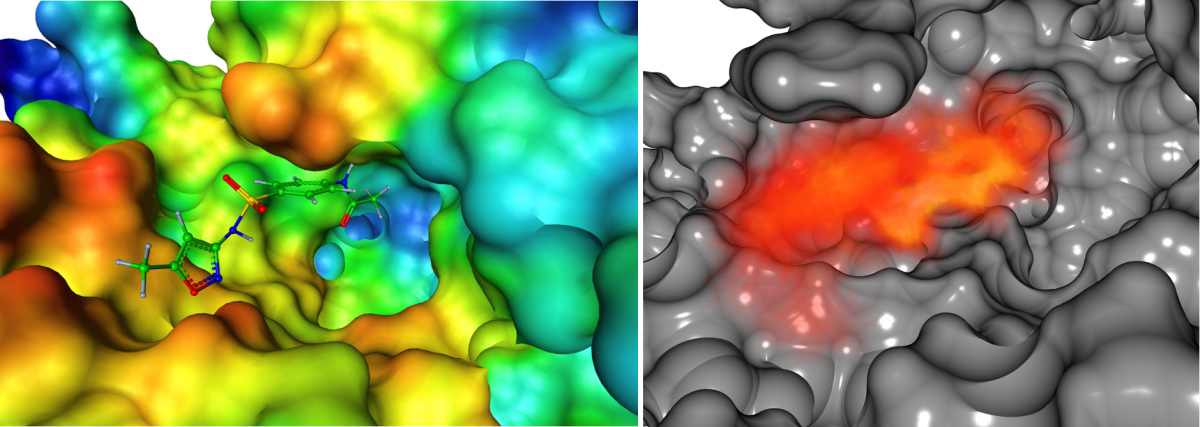
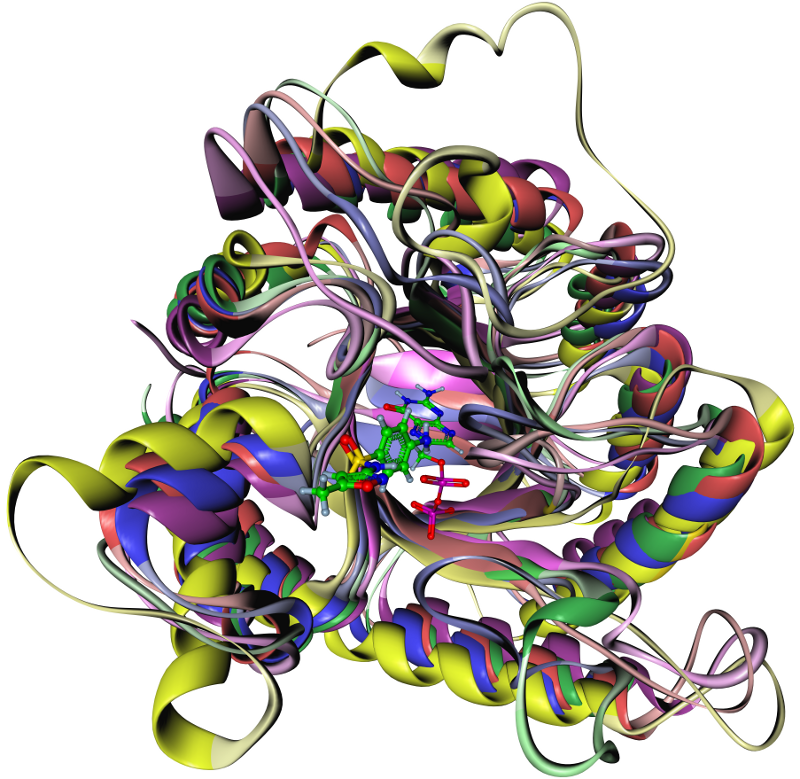
In light of these considerations we developed an molecular dynamics average-based model for prioritizing degradation products of the antibiotic sulfamethoxazole (SMZ) and the antiepileptic carbamazepine (CBZ) with respect to their binding affinity to its bacterial target dihydropteroate synthase (DHPS) and, respectively, the human metabolic enzyme cytochrome P450 (CYP). SMZ prevents DHPS from forming a precursor of folic acid that is essential for folic acid synthesis. Certainly, microorganisms tend to develop resistances against antibiotics when repeatedly exposed to them in sub-therapeutic trace amounts. In contrast, CBZ is not only metabolized by but also known to induce the activity of certain CYP variants. As a consequence, CBZ TPs might potentially affect human metabolism through a similar mode of action. For these reasons, it is of great importance to minimize environmental contamination with potentially bioactive anthropogenic substances. The task was to identify TPs of SMZ that are likely to mimic the DHPS-suppressive characteristic of SMZ. The colored figure below shows one TP of SMZ at the para-aminobenzoic acid (PABA) binding site of DHPS which is represented by its solvent excluded surface colored in accordance with its electrostatics.
The Gibbs free energy difference of binding ΔG for a guest-host-system describes the steady state distribution of bound and unbound ligands. ΔG is proportional to the dissociation constant Kd of the reversible binding process also denoted as binding affinity. Knowing the enthalpic contribution ΔH and the entropy difference ΔS at a certain temperature is sufficient for the determination of ΔG.
![]()
From our simulation data of the TP in both a protein-bound and unbound environment we are able to compute mean interaction energy (inner energy) differences for electronic (Eelec) and van-der-Waals (EvdW) interactions of the TP. Along with differences of the mean potential energy (ΔU) and the entropy (ΔS) we get very close the value of ΔG except for some weights xi that may be determined applying the linear interaction energy method and using a training set with known binding affinities to the target under consideration.
![]()
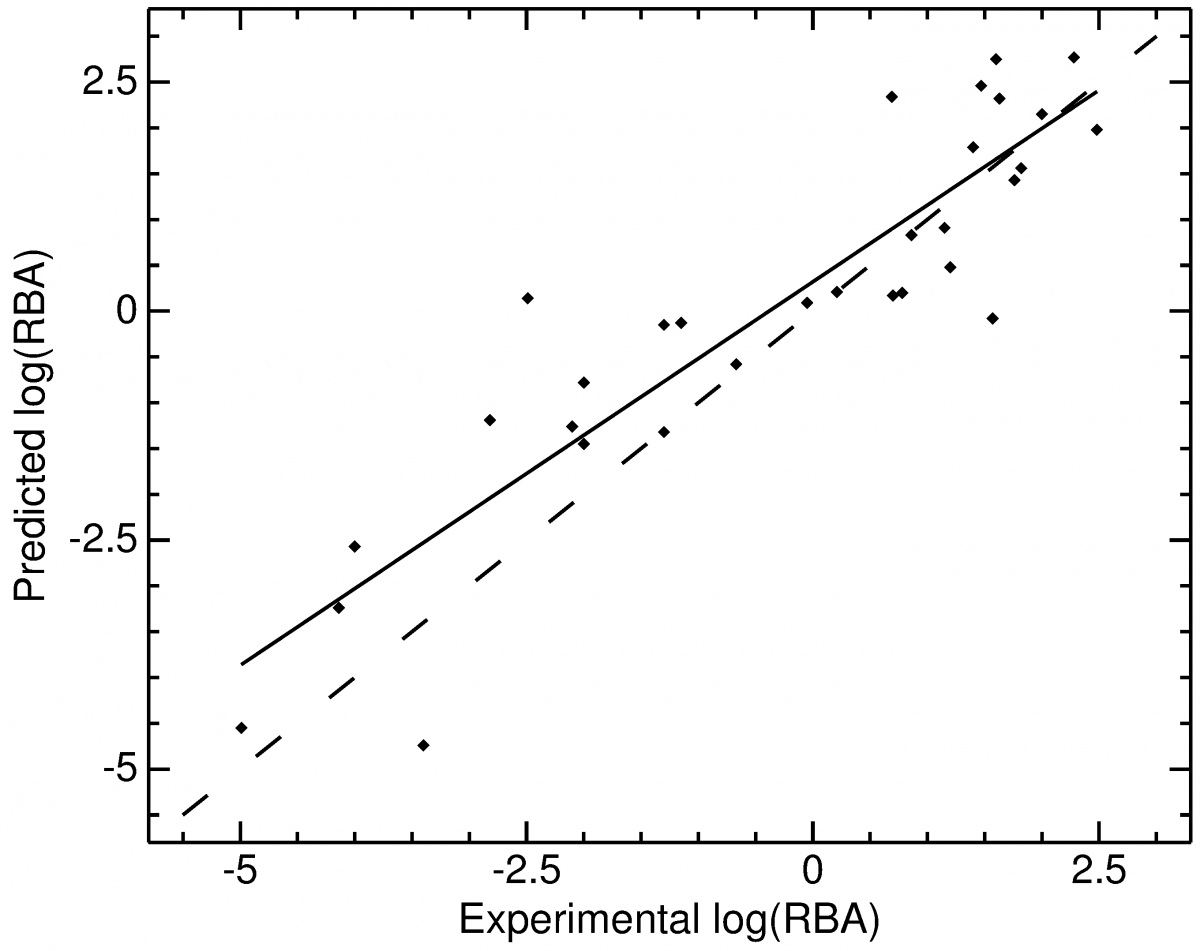
For a similar project, the estimation of estrogen activities, high correlations between predicted and experimentally estimated free binding energies have been computed. The cross-validation as well was very satisfactory.
But even without having trained parameter weights xi, one can quickly state whether a TP is supposed to have a higher affinity than its parent compound to a particular target if the values of all parameters are more favorable in terms of molecular interaction. The concept is illustrated by the following table:
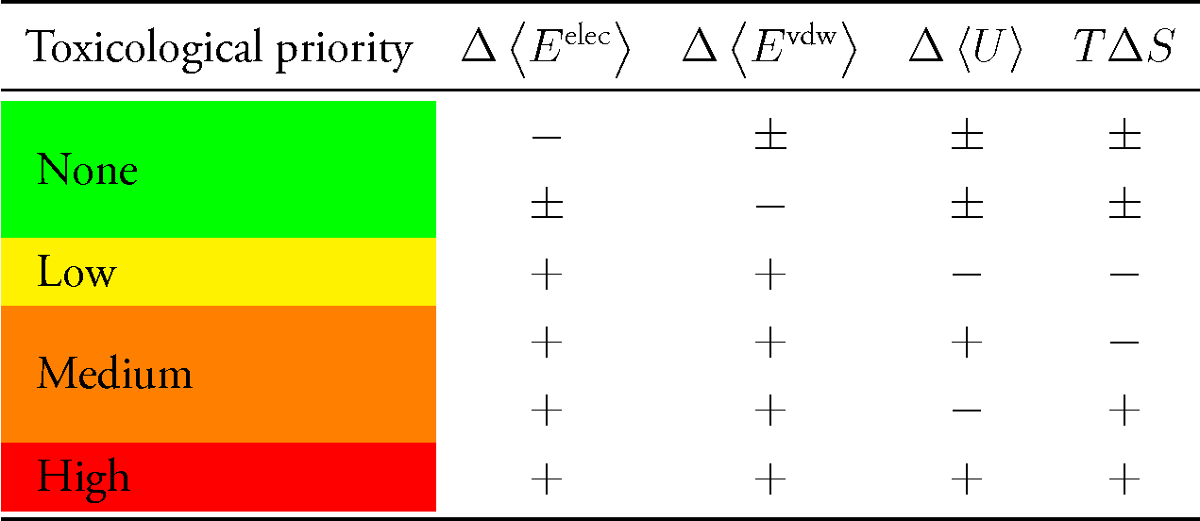
TPs are prioritized depending on the type and number of descriptors in favor of the TP rather than of the parent compound. An example is given in the table below regarding the SMZ TP denoted as N4-acetyl-SMZ.
| Free energy contribution | SMZ | N4-acetyl-SMZ |
|---|---|---|
| Δ<Eelec> [kJ/mol] | -7.25 | -13.69 |
| Δ<EvdW>[kJ/mol] | -44.40 | -48.71 |
| ΔU [kJ/mol] | 28.42 | 17.23 |
| ΔS [kJ/mol] | -5.27 | -2.67 |
Both interaction energy differences and the ligands potential energy difference are lower (more favorable) and the entropy loss upon binding is lower (more favorable) in case of this SMZ TP. Therefore, this certain TP of SMZ is supposed to bind stronger to DHPS of Staphylococcus aureus and was classified according to the highest priority. Five DHPS isoforms from different bacteria have been taken into account.
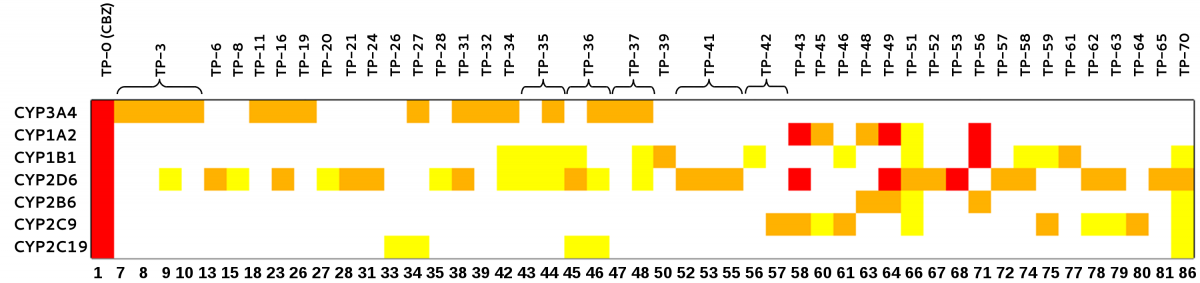
For the entire set of 30 SMZ TPs (40 diastereomers), the following graphic illustrates the relative estimated activities to the five DHPS isoforms. Legend: yellow (both interaction energy terms ΔE only indicating higher affinity than SMZ itself), blue (in addition to ΔE, the ligand's potential energy ΔU or conformational entropy ΔS indicating higher affinity), red (all four terms indicating higher affinity for the TP).
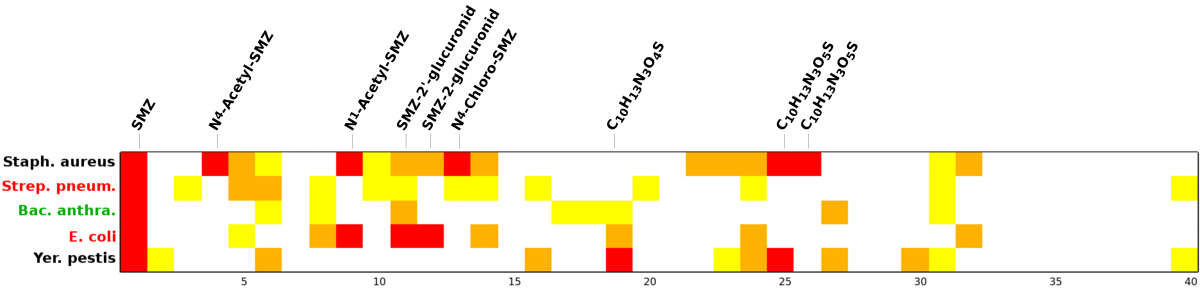
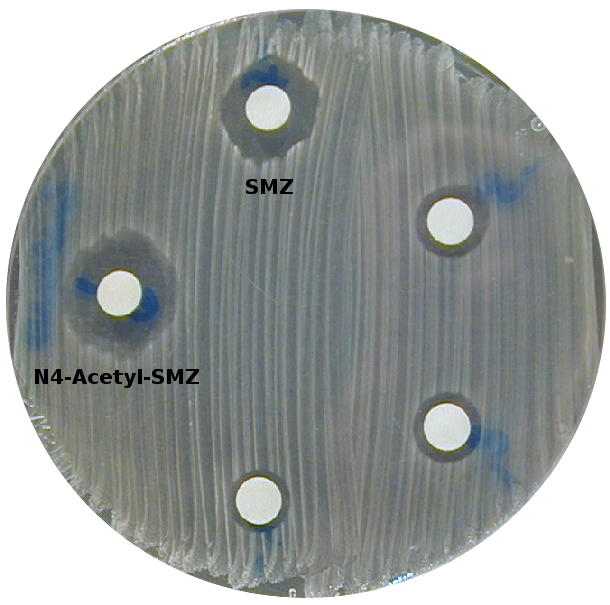
Alltogether, seven SMZ TPs of the highest risk category have been identified particularly affecting DHPS of the (partially) sensitive species S. aureus, E. coli, and Y. pestis. Some of those highest priority compounds were predicted to bind to several DHPS isoforms, but interestingly none of them seems to bind to the only resistant species under investigation, B. anthracis. According to our calculations, four high-priority metabolites are directed towards S. aureus of which critical multi-resistant strains are known that are hard to suppress with common antibiotics. Hence, one should greatly be interested in an as wide as possible elimination of SMZ as well as critical analogs already in properly upgraded sewage plants before they reach ground water. From this point of view, degradation products of the highest priority are strongly recommended for toxicological risk assessment through thorough laboratory tests. The only available agar diffusion test on the biotic SMZ metabolite N4-acetyl-SMZ reveals its suppressive effect on S. aureus growth which clearly confirms its predicted binding affinity in the order of or higher than SMZ itself.
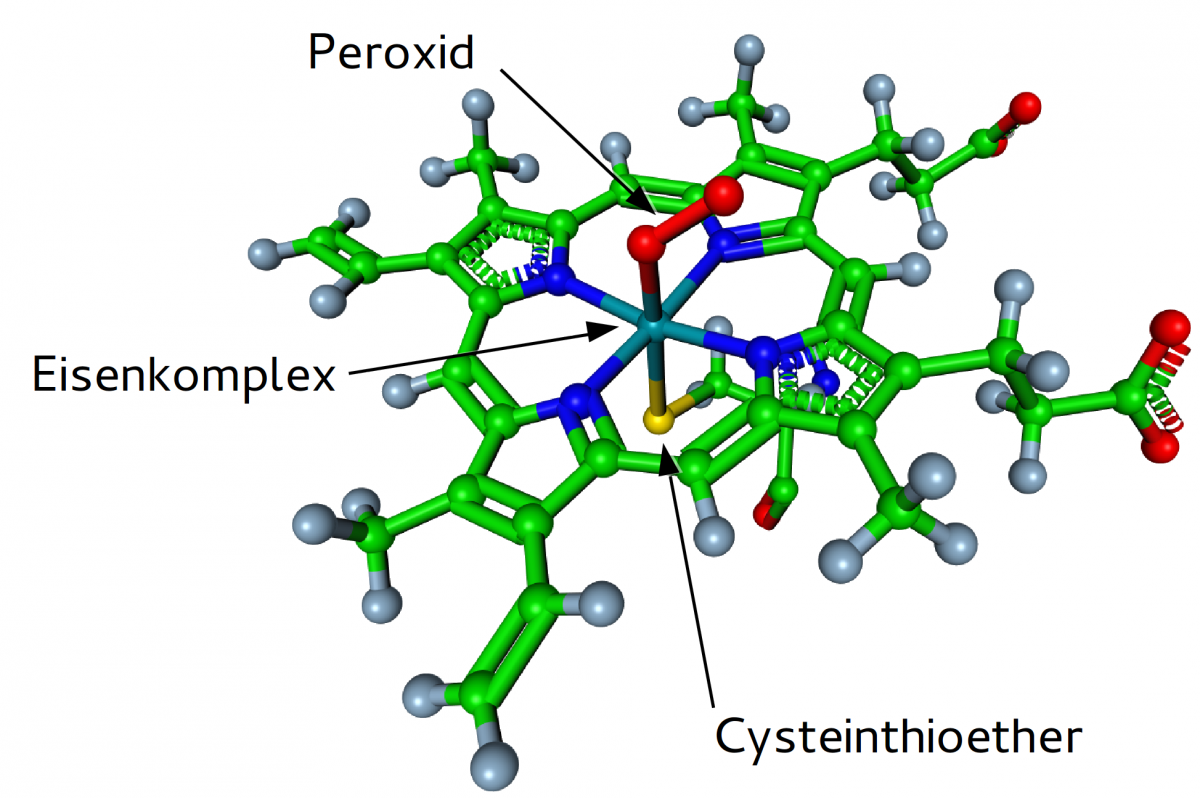
The same approach was made to TPs of CBZ with respect to several isoforms of cytochrome P450 (CYP) incorporating a HEM group that is particularly challenging when it comes to a parameterization for the purpose of empirical force field simulations.
For CBZ three TPs, 9-carbaldehyd-acridine (TP-43), iminoquinone (TP-49), and 9-carboxylic acid-acridine (TP-56), were identified which particularly reveal higher theoretical binding affinities to those CYP types that are known not only to metabolize but, moreover, to be induced by CBZ. For this reason, these CBZ TPs might affect human CYP-mediated metabolism as well and cause harm. Consequently, we strongly recommend to assess these metabolites by more reliable laboratory methods and eventually reduce their exposure to the environment.
In an additional investigation of the interaction of CBZ with the ecdysone receptor (EcR) of C. riparius was investigated. This protein is responsible for metamorphosis and reproduction and had been observed in an earlier study that CBZ inhibits C. riparius pupation. However, our results do not indicate any favorable mode of action and considerable binding affinity regarding this protein–ligand complex.