Understanding the function of the brain
Basic neurobiological research aims to investigate the structural composition, function, and development of biological neural networks (including the human nervous system). This research lays the foundation for a better understanding of the development of neurological diseases, such as Alzheimer’s disease, and enables the development of targeted medications and therapies for mental disorders and neurological diseases.
Neurobiological research, like many of today’s research fields, is extremely diverse and conducted on various levels. Although understanding the human brain is a primary goal, the possibilities of directly examining the brain at the cellular level are severely limited due to the non-invasive imaging techniques available. In smaller organisms, on the other hand, it is possible to examine the structure of the brain in much greater detail or even completely at the cellular level by reconstructing individual nerve cells (neurons) and their synaptic connections using modern imaging and mathematical image processing techniques. Basic neurobiological research is therefore usually conducted on various types of animal, such as fruit flies, zebrafish, mice or rats.
At the Zuse Institute Berlin, the “Visual Data Analysis” research group has been conducting intensive research with neurobiologists in various ways for many years. The goal in these projects is to support the work of neurobiologists through state-of-the-art techniques of mathematical image analysis and visualization. The following three projects highlight some aspects of these collaborations.
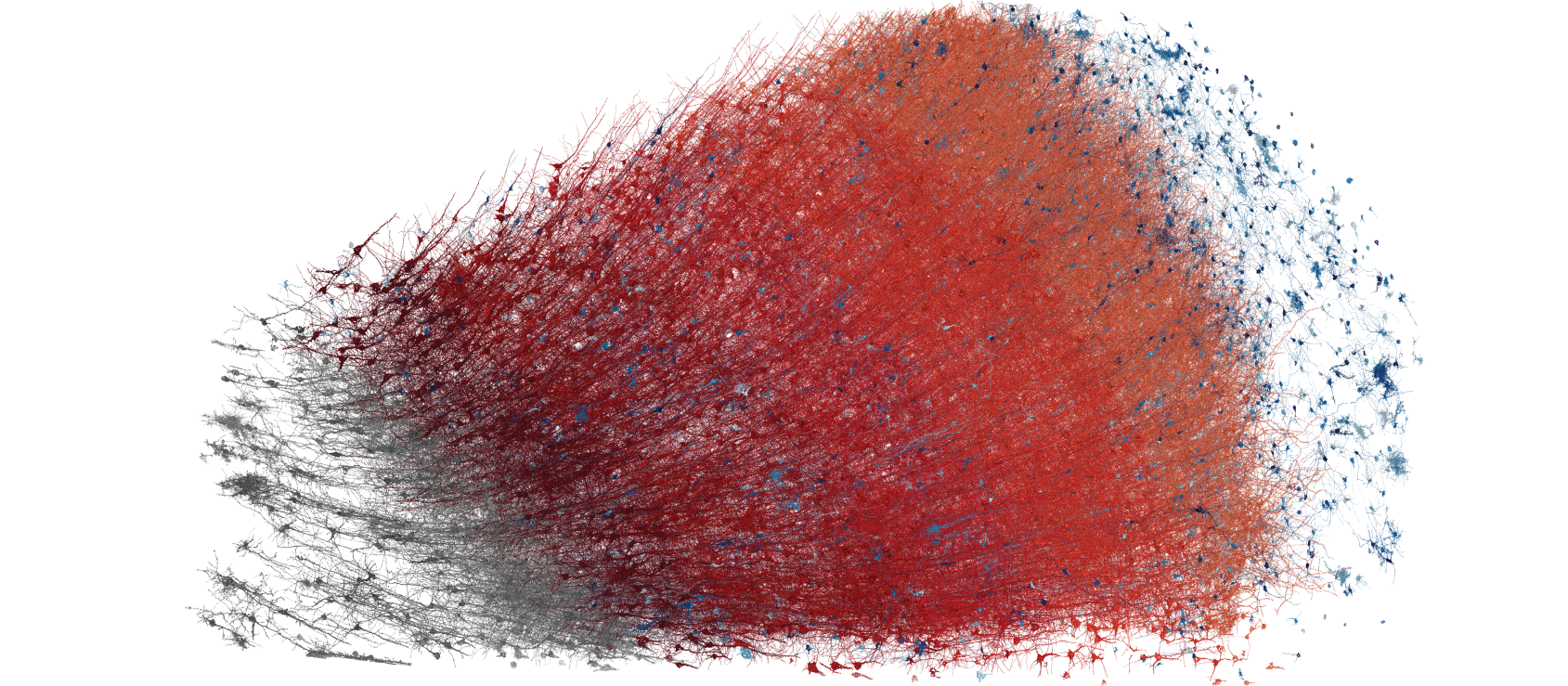
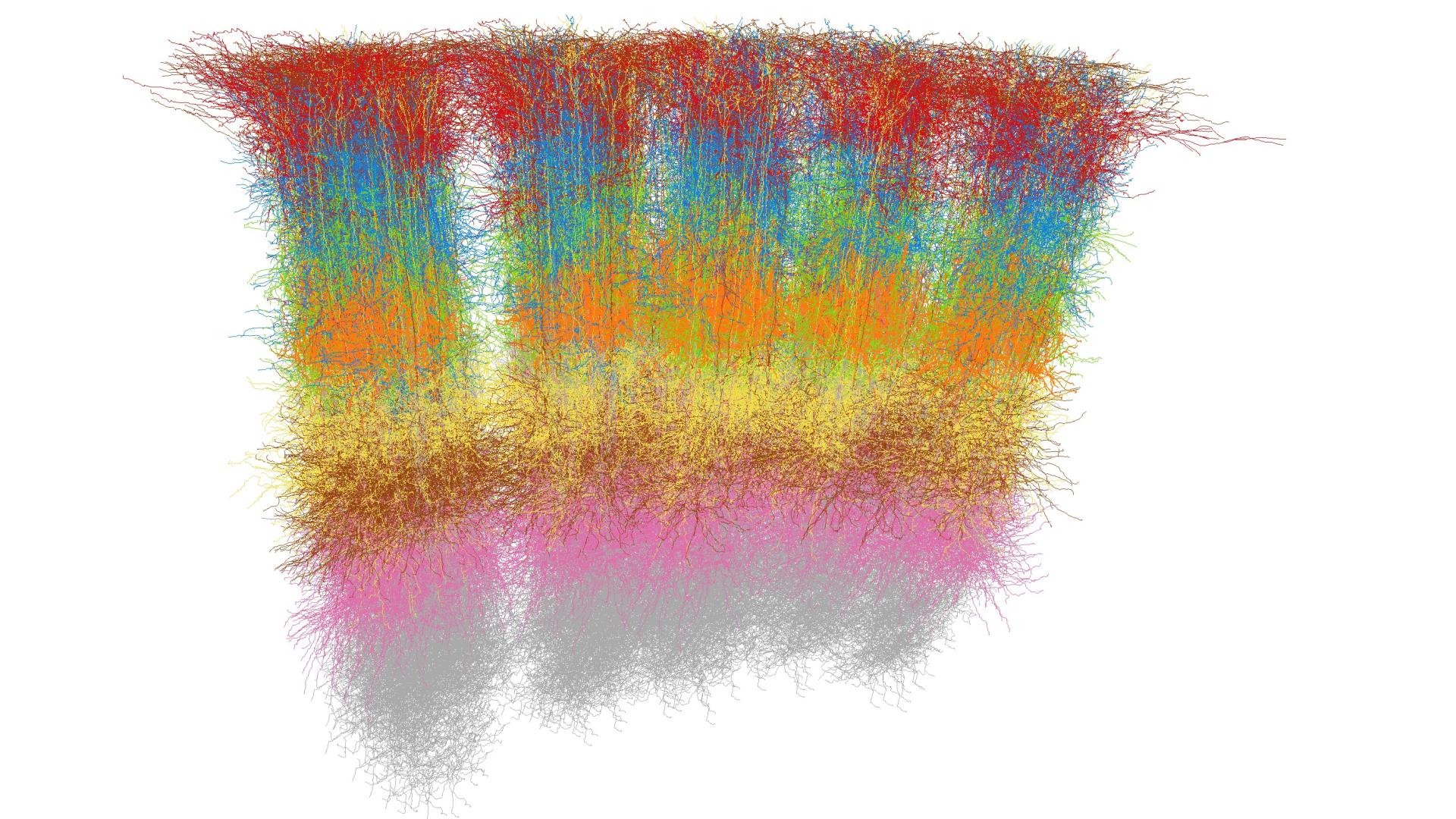
Figure 1: Electron microscopy reconstruction of a human cortex tissue sample consisting of around 16,000 neurons. Pyramidal cells are shown in red, shaded by the cortical layer; interneurons are shown in blue. Data: Shapson-Coe et al. 2024; visualization: Amira.
Within the DFG Research Unit “RobustCircuit”, led by Prof. Hiesinger from the Free University of Berlin (FU), neurobiologists from FU and the University of Mainz are investigating the role of noise – in the sense of random fluctuations – in the development and function of the brain on various spatial and functional scales. For this purpose, three-dimensional (3D) images and movies are acquired that allow one to see how the brain develops. Our task in this research unit is to develop automated techniques to analyze these data, for example, to extract statistical data about how the neurons grow, how they interact, and what molecular factors influence their growth. By automating these analysis tasks, we enable neurobiologists to analyze an unprecedented amount of data to derive robust statistical conclusions that would otherwise be infeasible.
In cooperation with Prof. Engert from Harvard University (Boston, USA), we are working on the creation of brain atlases and their use for behavioral studies as well as on the comparison of brains from different species and animal groups. For this, we have developed a web-based platform that allows the integration of the original image data, extracted structural data (brain regions and neurons), and functional data (neurons activated in behavioral experiments and gene expression data) into a common coordinate system. As a neurobiological model system, this project uses the zebrafish, whose larva is fully transparent for a few days after hatching and is therefore particularly suitable for capturing functional data. To complement the atlas, we have developed advanced visual analysis tools that enable neurobiologists, based on functional measurements taken during behavioral studies, to identify hypothetical neural circuits that are responsible for performing specific functions in response to certain visual, acoustic or tactile stimuli.
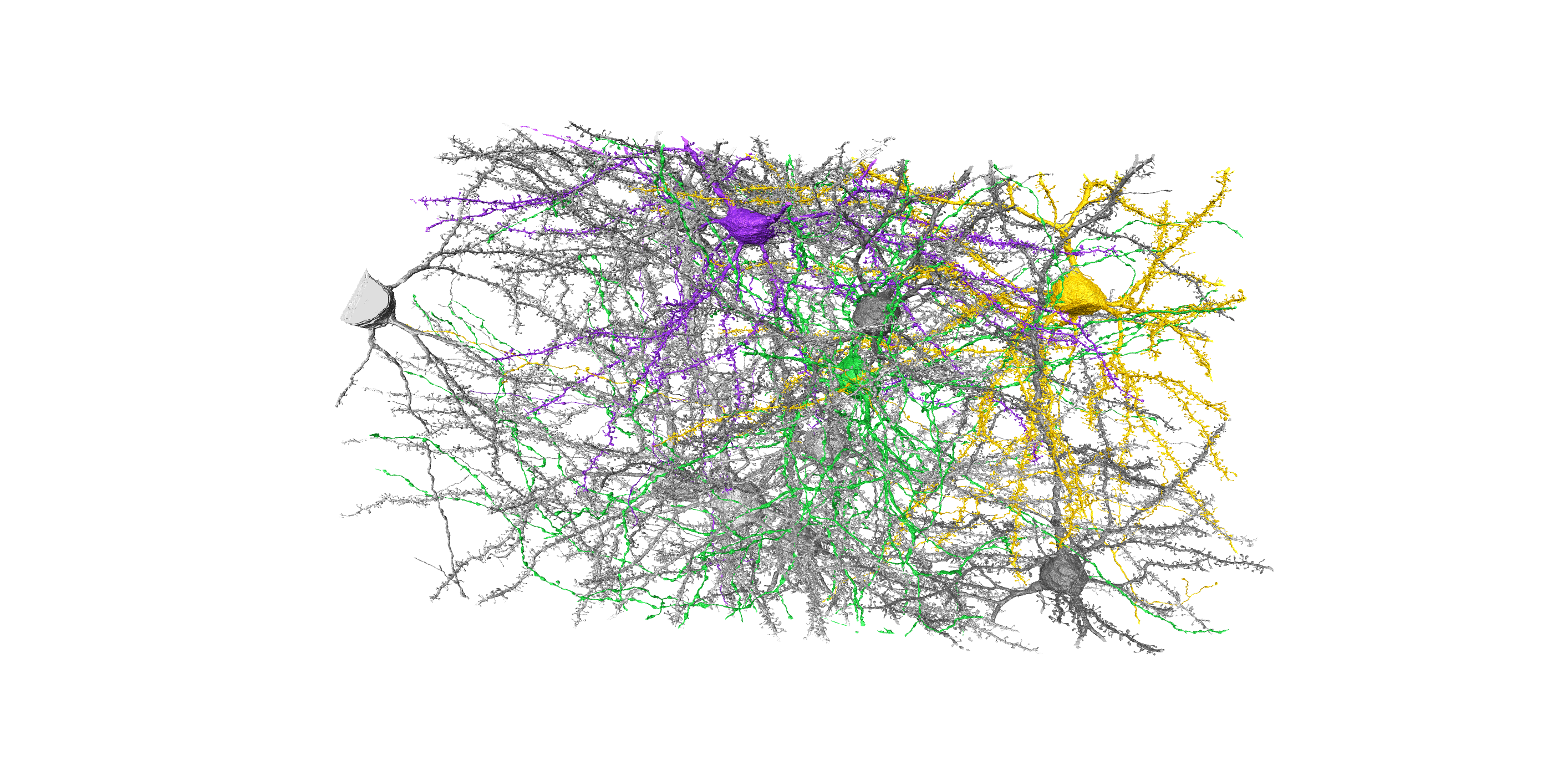
Figure 2: Neuronal cells from the visual cortex of the mouse, forming a dense network. Three neurons are highlighted in color to show the highly branched structures (axons and dendrites) that innervate the surrounding tissue from the cell nucleus (soma). Data: Turner et al. 2022; visualization: Amira
In a third project, in collaboration with Prof. Oberlaender from the MPI for Neurobiology of Behavior (caesar), Bonn, and Prof. Macke from the University of Tübingen, we developed analysis tools for the examination of connectome data, which represent the synaptic connections between neurons. Using simulation-based Bayesian inference, we developed a generative mathematical model that, with very few parameters, was able to reconstruct the structural properties of various connectomes. With this innovative technique, we can now for the first time generate network models direct-ly from dense electron microscopy data that capture features of empirically observed connectomes from subcellular to network scales, and that, at the same time, enable the derivation of the synaptic specificity parameters that are necessary and sufficient to explain each of these connectivity features. With the help of our model, surprisingly, we identified strong similarities in the synaptic specificity parameters for the mouse visual cortex and human temporal cortex.