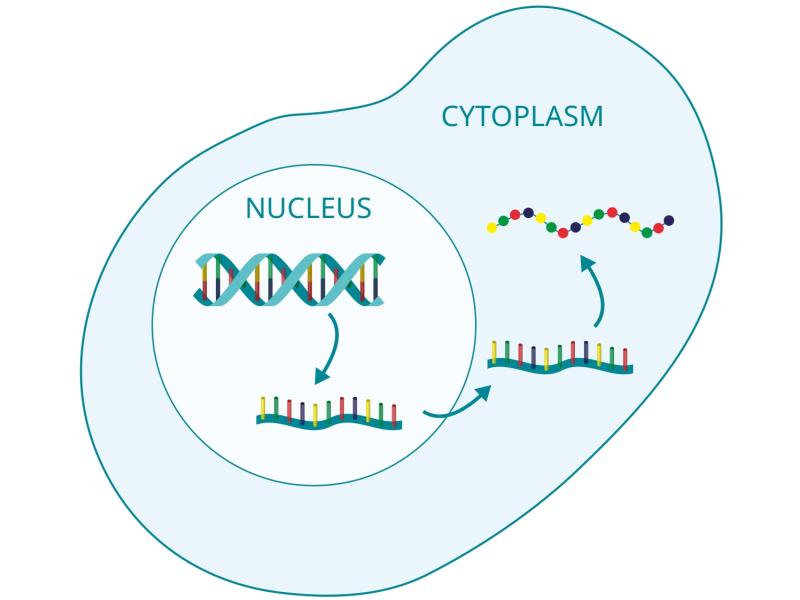
In this project we analyze various techniques for modeling spatio-temporal cellular processes with cascades of scales in time, space and population size. We successfully constructed several new hybrid models that seamlessly couple different resolutions for different molecular species and spatial locations within the cell, e.g., integrating particle-based reactions diffusion (PBRD) models with spatio-temporal master equations and/or stochastic partial differential equations, or coupling PBRD models with molecular kinetics like conformational changes or membrane dynamics. Efficient algorithms for numerical simulations were proposed and their superior performance (in comparison to non-hybrid models) was demonstrated for various real-life applications.
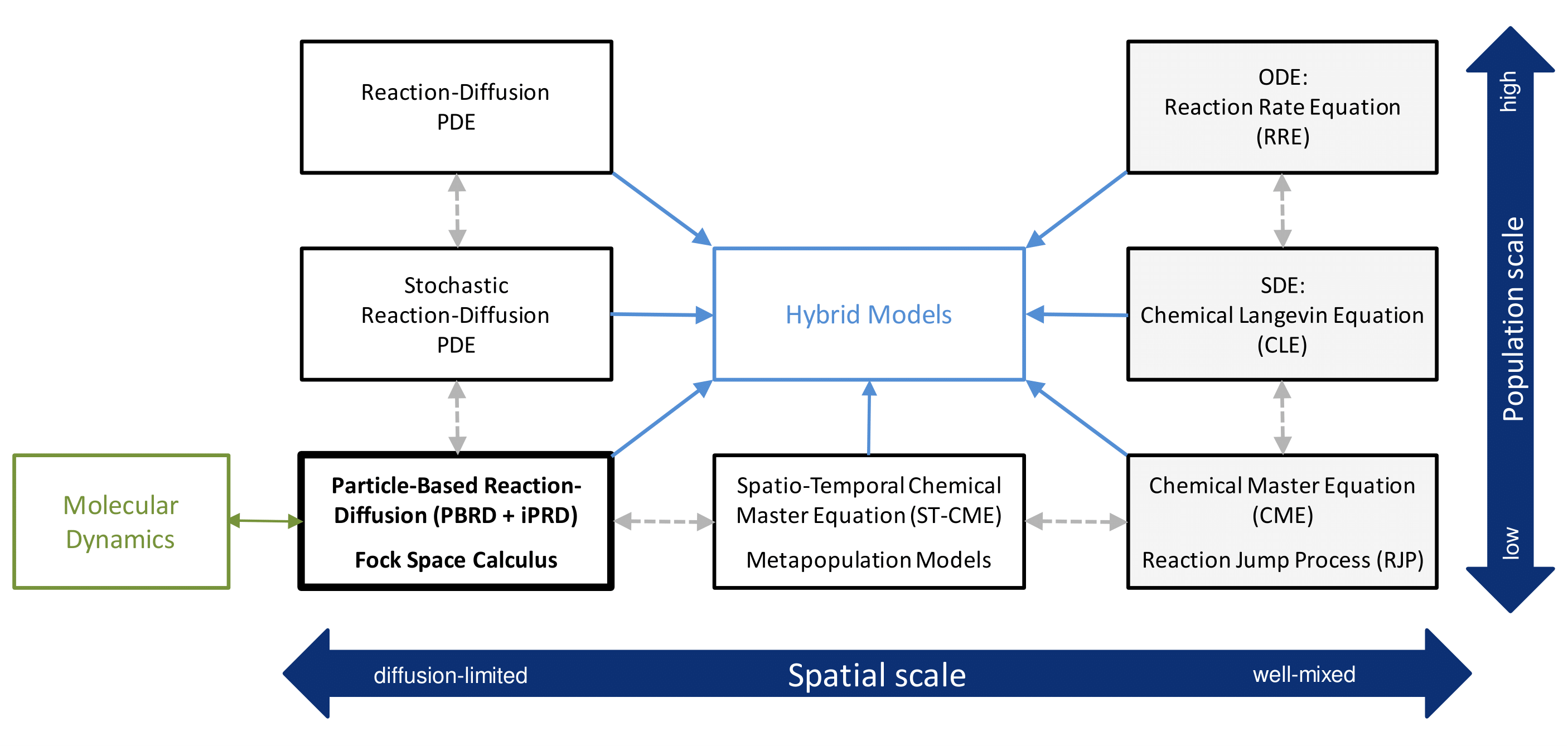
Figure 1: Overview of mathematical modeling approaches for biochemical reaction-diffusion kinetics.
In the third funding period we refine our methods further and integrate external progress in the field in order to construct a hybrid model and appropriate simulation algorithms for investigating the process of receptor diffusion, co-localization, and kinetic interaction on the membrane surface of neurons. For this process, brand-new experimental data with single-molecule resolution in time and space is available that indicates surprising cluster formation of receptors [1]. Modeling this process requires coupling of receptor conformation kinetics with diffusion, mediated by the interaction with the membrane, and bi-molecular interaction, making it into a prototype for the interaction across a cascade of scales.
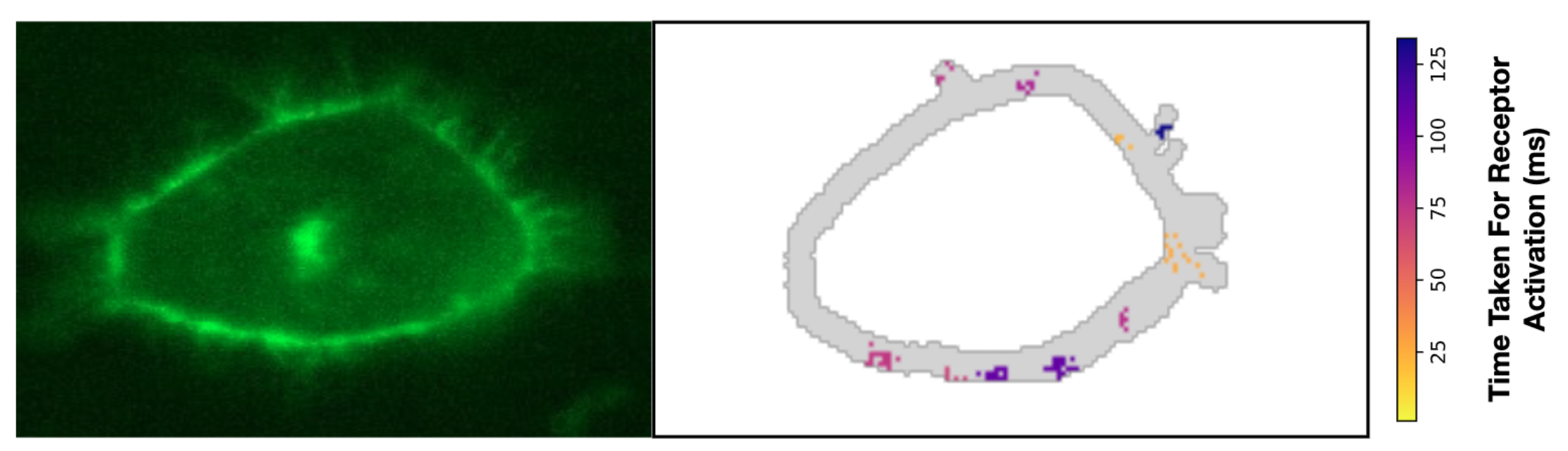
Figure 2: Cluster formation in receptor activation on the surface of living cells [1].
References
[1] J. Möller, A. Isbilir, T. Sungkaworn, B. Osberg, C. Karathanasis, V. Sunkara, E. O. Grushevsky, A. Bock, P. Annibale, M. Heilemann, C. Schütte, and M. J. Lohse. Single-molecule analysis reveals agonist-specific dimer formation of μ-opioid receptors. Nature Chemical Biology, 16:946 – 954, 2020.
[2] M. Sadeghi and F. Noé. Thermodynamics and kinetics of aggregation of flexible peripheral membrane proteins. The Journal of Physical Chemistry Letters, 12(43):10497–10504, 2021.